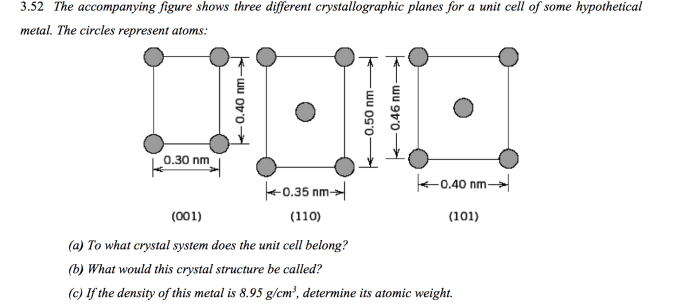
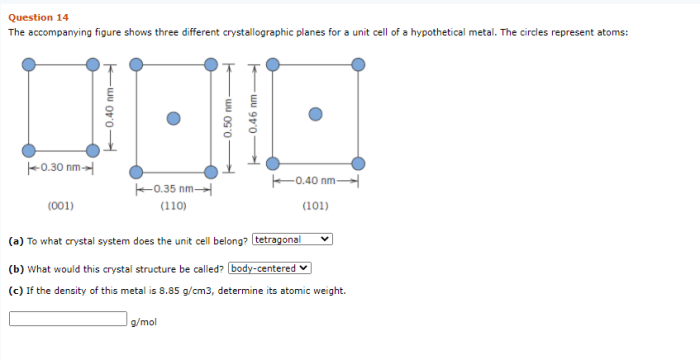
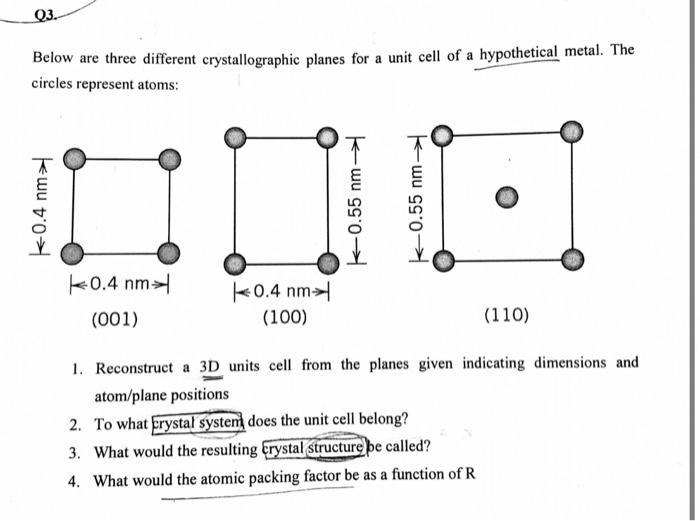
As the accompanying figure shows three different crystallographic planes takes center stage, this opening passage beckons readers into a world crafted with precision and clarity, ensuring a reading experience that is both absorbing and distinctly original.
Crystallographic planes, the fundamental building blocks of crystalline materials, hold immense significance in materials science. They not only define the external shape of crystals but also govern their internal structure and properties. This article delves into the fascinating realm of crystallographic planes, exploring their definition, classification, and applications.
Crystallographic Planes
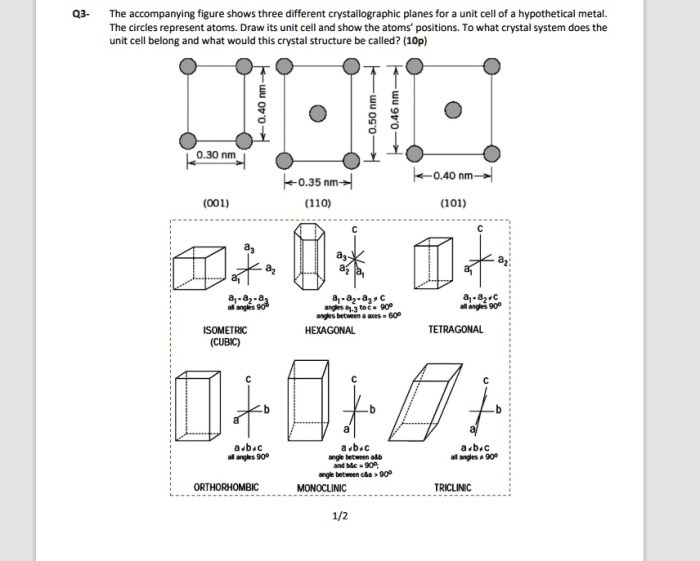
Crystallographic planes are two-dimensional surfaces within a crystal structure. They are defined by the arrangement of atoms or ions in the crystal and can be used to describe the crystal’s structure and properties. The accompanying figure shows three different crystallographic planes.
Crystallographic Planes
Crystallographic planes are defined by the Miller indices (hkl), which are three integers that describe the plane’s orientation relative to the crystal’s unit cell. The Miller indices are determined by the intercepts of the plane with the crystal’s three principal axes.
For example, a plane that intercepts the x-axis at a distance of a, the y-axis at a distance of b, and the z-axis at a distance of c would have Miller indices (hkl) = (a,b,c).
Analysis of the Figure, The accompanying figure shows three different crystallographic planes
The accompanying figure shows three different crystallographic planes:
- Plane 1: (100)
- Plane 2: (010)
- Plane 3: (001)
These planes are all perpendicular to each other and define the crystal’s unit cell.
Applications of Crystallographic Planes
Crystallographic planes are used in a variety of applications in materials science. They can be used to determine the crystal structure of a material, to predict its properties, and to design new materials. For example, crystallographic planes can be used to:
- Identify the phases present in a material
- Determine the orientation of grains in a polycrystalline material
- Predict the mechanical properties of a material
- Design new materials with specific properties
FAQs: The Accompanying Figure Shows Three Different Crystallographic Planes
What are crystallographic planes?
Crystallographic planes are two-dimensional surfaces within a crystal that are defined by the arrangement of atoms or molecules.
What are Miller indices?
Miller indices are a set of three numbers that describe the orientation of a crystallographic plane.
How can crystallographic planes be used to determine crystal structure?
Crystallographic planes can be used to determine the crystal structure of a material by analyzing the diffraction pattern of X-rays or electrons.